The functionalities of organs and tissues are tightly linked with their structure and, the organisation of constituent cells. Cues from the surrounding extracellular matrix direct the orientation and alignment of cells with respect to one another through contact guidance [1]. This microenvironment is crucial in dictating cell fate; affecting morphology, proliferation, differentiation, response to stimuli, subcellular composition and protein synthesis among others [2,3]. It is important to recreate these interactions as accurately as possible when developing physiologically relevant in vitro models as an alternative to animal testing.
As part of the RENOIR European Training Network, researchers aim to develop a microfluidic cell culture device to model the vascularised muscle niche, and investigate the effects of aging and disease on muscular regeneration.
The reciprocal relationship between structure and function observed is an important consideration when developing in vitro culture models. To this end, various microfluidic designs and strategies have been applied to spatially organize and align cells in culture devices, including mechanical stimulation, surface topographical features, and chemical surface treatments [4]. This article will discuss these methods with a particular focus on devices for the study of skeletal muscle and vascular tissues, where cellular organisation must be precise.
The Structure-Function Relationship in Tissues
Skeletal Muscle Tissue
Skeletal muscle is made up of rod-shaped multinucleated myotubes formed through the fusion of aligned myoblast cells. The myotubes are organised in parallel along the contraction axis, creating muscle fibres which extend the full muscle length [5]. At the subcellular level, actin and myosin cytoskeletal filaments are also arranged along this common axis [4]. This comprehensive structure is essential in generating a contractile force to achieve the main function of skeletal muscle – moving the body (Figure 1A).
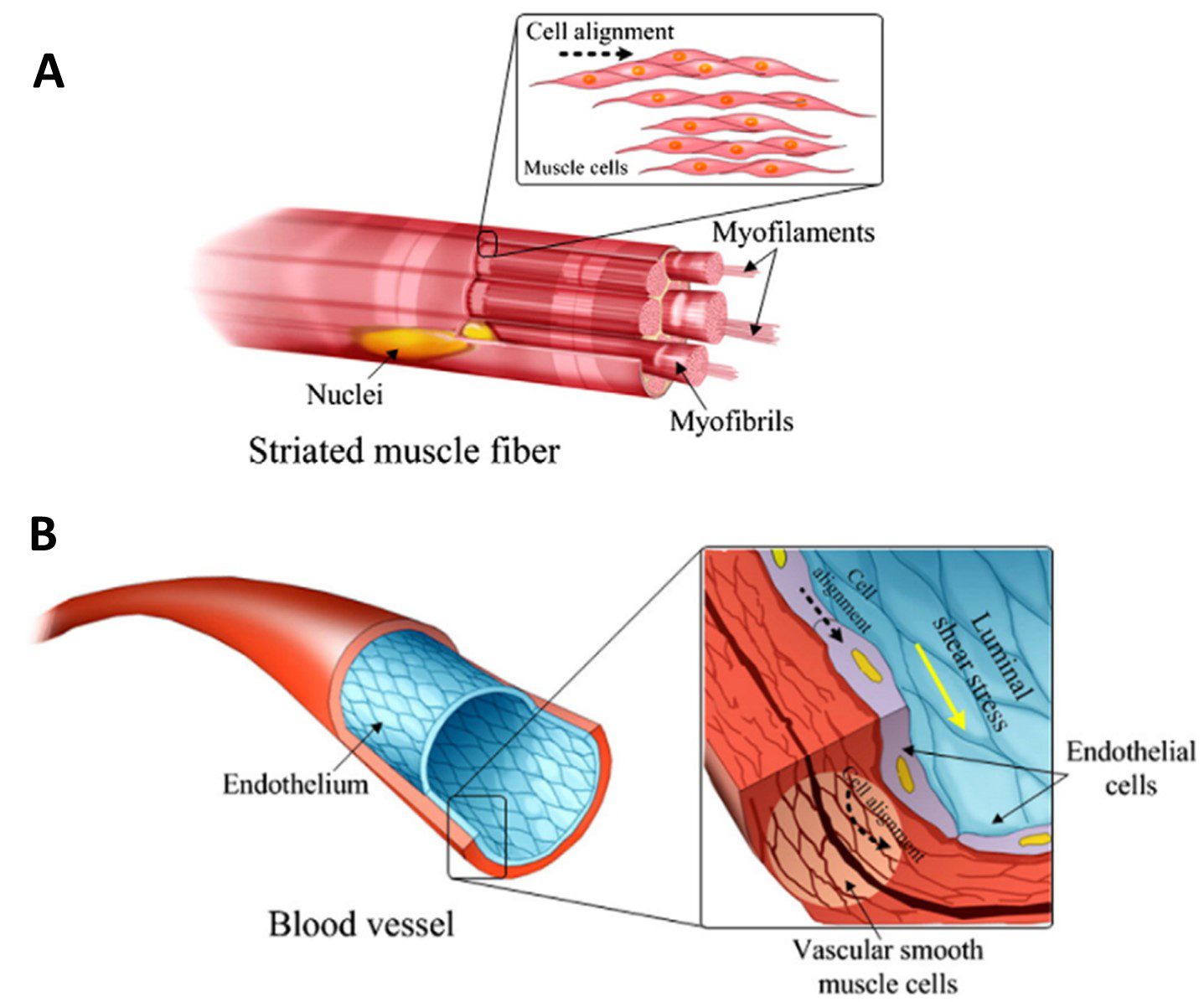
Figure 1. Cellular organisation and alignment in A) blood vessel and B) striated muscle fibre
Vasculature
Blood vessels are comprised of various cell types, the arrangement of which makes them able to withstand fluid shear stress imposed by constant blood flow.
A helix of smooth muscle cells and pericytes are arranged around the outer vessel circumference, providing flexibility and tensile strength which contributes to the resilience of the tissue under pulsatile and high-pressure fluid flow [4]. On the inner surface, a lining of endothelial cells is arranged along the longitudinal axis of the vessel. They are elongated and polarized along the direction of blood flow [6]. Similar to skeletal muscle, the intracellular cytoskeleton filaments reflect this same alignment (Figure 1B).
Cell Alignment by Mechanical Stimulation
To recreate the above structures inside of a 3-dimensional microfluidic environment, different techniques are being developed. One such method is mechanical loading, which encourages a specific architecture within a cell culture. When considering muscle and vascular tissues, the effects of stretching and fluid flow shear stress are of most relevance with regards to their functions in vivo.
Stretching
Many tissues experience periodic mechanical stretching in vivo. Skeletal muscle contract and relax to generate movement and blood vessels experience stretching through pulsatile blood flow. Cells within these tissues, such as fibroblasts, endothelial cells and smooth muscle cells, are sensitive to mechanical load.
However it is a practical challenge to apply stretch to a cell culture. The growth membrane must be a stretchable material and the external set-up must allow a uniform uniaxial strain to be applied. Parameters of stretch direction, magnitude, duration, and cycle frequency must be considered [4].
Stretchable membranes have been integrated into microfluidic culture devices to recapitulate physiological movements of tissues [7]. Peristalsis was recreated in a gut-on-chip model by Kim et al. by applying negative air pressure to vacuum channels flanking a central culture chamber, causing the chamber to deform and stretching the PDMS membrane upon which the cells were growing [8] (Figure 2A). In a recent lung-on-chip model, the flexing of alveoli during breathing was mimicked by applying a vacuum to stretch a thin collagen-elastin membrane through a meshed structure [9] (Figure 2B). This is a great advantage microfluidic models can deliver over classic 2D cell culture methods.
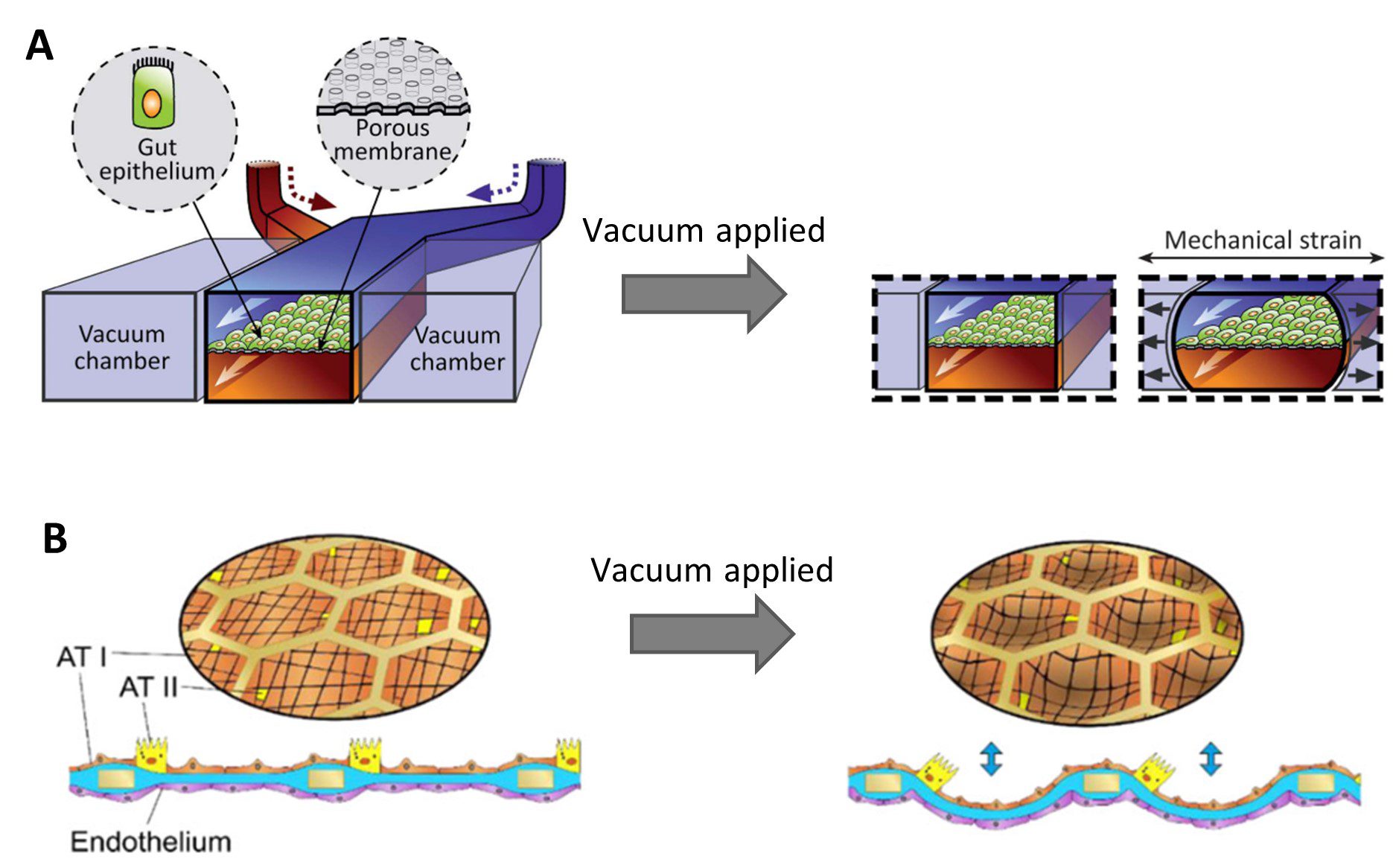
Figure 2. Stretching culture membranes within microfluidic devices by applying vacuum in order to recapitulate A) peristalsis in gut-on-chip [8]; B) alveolar flexing in lung-on-chip [9]
Fluid Flow Shear Stress
As briefly mentioned, hemodynamic forces experienced by endothelial cells in blood vessels influences their morphology and alignment, including cytoskeleton organisation. In areas of high shear stress, endothelial cells have more stress fibres when compared to cells in regions of lower shear [6]. These fibres are oriented along the direction of flow. This allows them to withstand the high shear rates and reduce cellular damage.
In order to induce alignment using fluid flow shear stress, the flow profile should be laminar and unidirectional. The magnitude of shear stress on aligned arterial endothelial cells in vivo is within the range 10 – 70 dyne/cm2. The exposure time, the magnitude of shear, and the cell type affect the response [10].
Kohn et al. (2015) showed alignment and elongation of endothelial cells parallel to flow direction after 24 hours exposure to 12 dyn/cm2 shear stress, shown in Figure 3[11]. This corresponds to the in vivo organization of endothelial cells in blood vessels (Figure 1B).
Fluid flow shear stress is often used as a method of physical stimulation in microfluidic devices due to the laminar flow and high control over the fluid flow profile, making it a predominant technique in the development of vasculature-on-chip models [12,13,14].
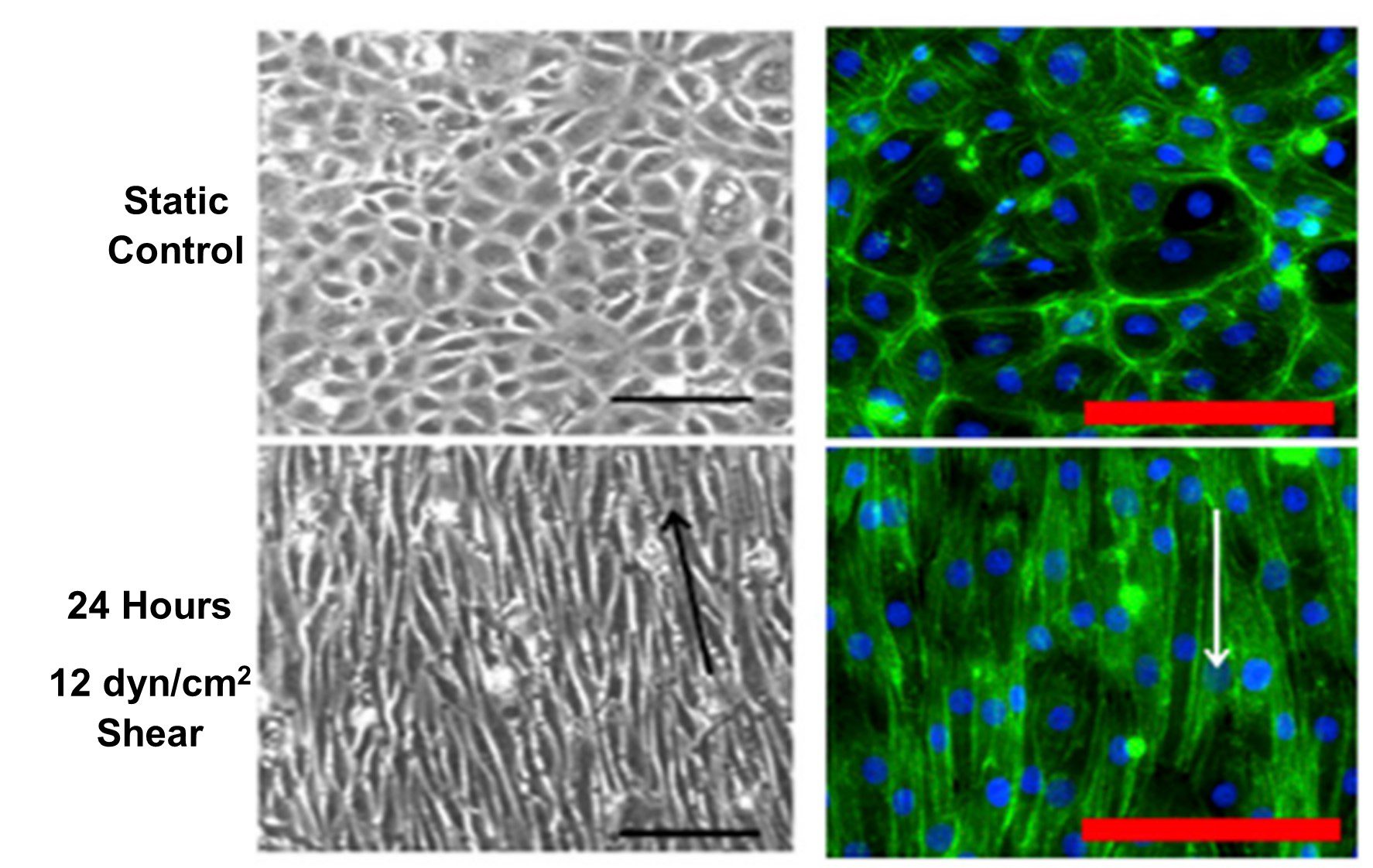
Figure 3. Under exposure to fluid flow shear stress endothelial cells align and elongate parallel to the flow direction (indicated with arrows). Immunofluorescent staining for f-actin (green) and DAPI (blue) on the right side and phase contrast on the left. Red/black scale bars = 100 µm (Kohn et al., 2015)
Cell Alignment by Surface Topography
The topography of the growth substrate surface can also be used to manipulate the distribution and positioning of cells. Microscale grooves, pillars and pits are some physical microfeatures which can be integrated into microfluidic cell culture devices to encourage specific tissue architectures
Grooves
The integration of parallel, repeating grooves on substrate surfaces have been used to direct cell growth. Being of similar size to a single cell, microscale grooves induce alignment by providing attachment points for cells [4]. This guides cell positioning along the groove direction, encouraging aggregation in structured lines. Altering the groove dimensions (i.e. depth, width, and spacing) leads to changes in cell positioning, depending on the cell type and desired architecture of the culture [15]. The orientation of the grooves with respect to the flow direction must be also considered. Anene-Nzelu et al. cultured myoblasts in microfluidic channels with grooved surfaces both perpendicular and parallel to the flow direction. Both orientations showed multi-layered, organised constructs (with different geometries) when compared to the smooth channel surface (Figure 4)[16].
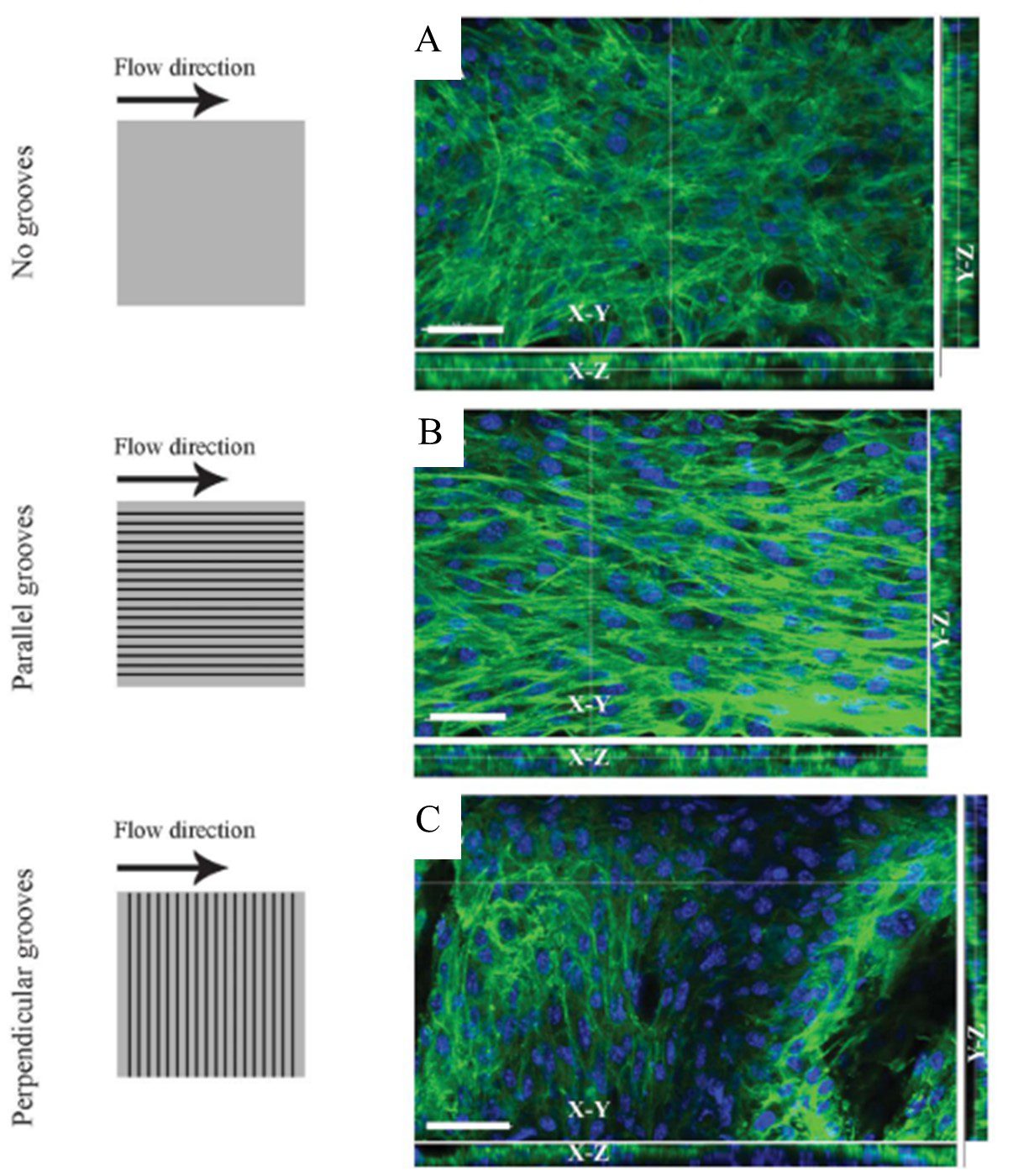
Figure 4. C2C12 myoblast cells after 3 days of culture within grooved channels of a microfluidic chip. Immunofluorescent staining for f-actin (green) and DAPI (blue) revealed aligned 3D constructs in the grooved channels at both parallel (B) and perpendicular (C) orientations to the flow direction when compared to the random cell positioning observed on the smooth channel surface (A). Grooves of dimension 30 x 50 µm, with 20 µm spacing. Scale bar = 50 µm. [16]
Pits
Patterned arrays of pits can be used to guide the cell positioning on the substrate surface. A comparison of grooves and pillars by [15] showed that both influenced cell positioning, grooves tended to lead to a uniform parallel cell alignment whereas cells grown within the pillar array were preferentially positioned horizontally and vertically between the structures (Figure 5). As is the case for the other topographical features, the dimensions and spacing of the indentations are important in dictating the alignment of cells in the culture [17].
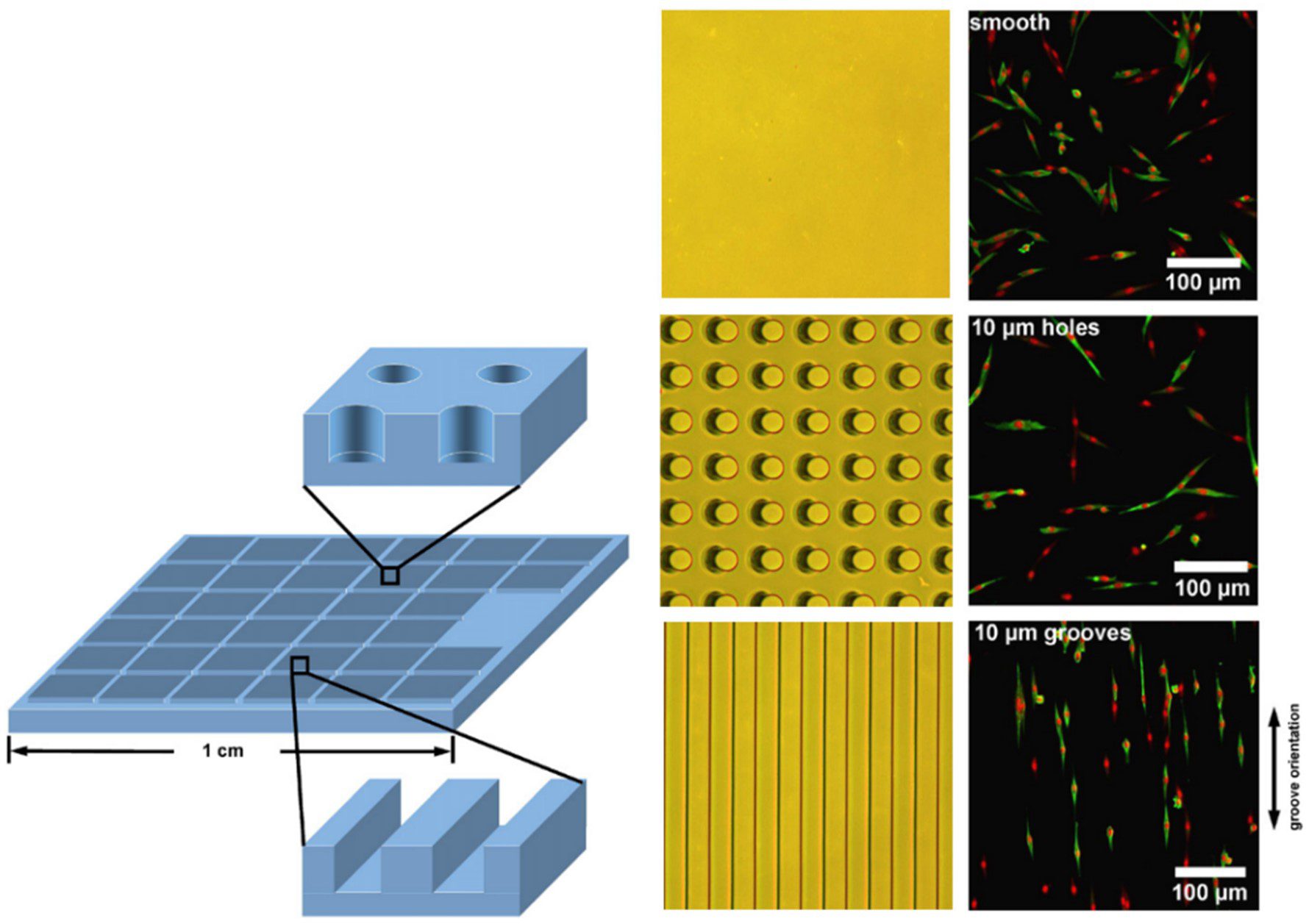
Figure 5. Growth substrate with engineered 10 µm wide grooves and 10 µm deep pits induced alignment of primary myoblasts, shown immunostained for sarcomeric myosin (green) and nuclei (red). Cells are positioned randomly on the smooth control surface, are directed horizontally and vertically on the pitted surface, and align parallel to the groove direction on the grooved surface [15].
Pillars
Similar to pits, pillar arrays have been included in cell culture devices to influence cell positioning. The geometries and spacing of the pillars have an effect on the cell response and are dependent on the specific cell type [18]. Micropillars have also been employed as anchor points where cells can be grafted and encouraged towards a specific positioning [19].
Cell Alignment by Chemical Surface Treatment
Surface functionalization is an important consideration in microfluidic devices. Different material surfaces can be patterned with chemicals to selectively position cells on the substrate. This can be achieved with chemicals that either facilitate or inhibit cell adhesion. For example, fibronectins and laminins promote the formation of focal adhesions encouraging cell attachment through integrin binding (Figure 6).
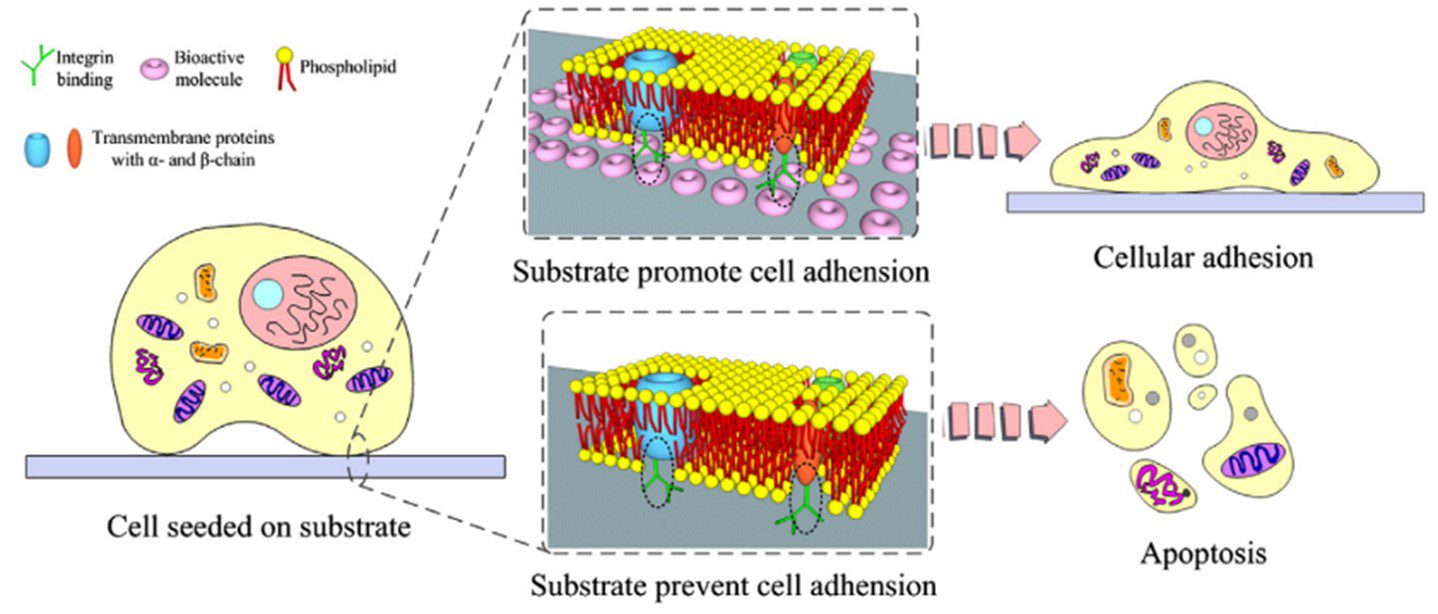
Figure 6. Chemical patterning for selective cell adhesion by either facilitating integrin binding of cells to promote adhesion to the substrate or prevent adhesion with chemicals that cause apoptosis [4].
Chemical surface patterning can also be used in combination with other alignment methods. Ahmed et al. (2010) combined cyclic stretching with parallel lines of cell-adhesive fibronectin to align myoblasts. Grooves with functionalised surfaces were investigated by Charest et al. (2006), and Lam et al. (2012) applied fluid flow shear stress to endothelial cells cultured in micropillars with chemical surface patterning [15,21].
Challenges for Application of Cell Alignment Techniques
Applications in 3D cell culture
These methods have all shown success in inducing cell alignment, but are only well investigated in monolayer cultures. Therefore, they fall short in recapitulating the full complexity of tissues in vivo. So 3D cell culture methods have emerged to provide a more physiologically relevant model of tissue microenvironments. Organ-on-Chip devices are at the forefront of 3D cell culture methods, and so it is important to translate these alignment methods for use in microfluidics. The miniaturization of experiments using microfluidics has a multitude of advantages for both industry and academia. It provides compactness and portability, for infrastructure-limited scenarios. It diminishes the volume of reagents required, which can be precious or expensive. The small scale also means the experimental analysis and reactions occur at a shorter timeframe. There are microfluidic devices with multiple functions, providing innovators with the ability of automating and integrating these together, reducing the probability of contamination and improving safety. Consequently, microfluidic technology has become an exciting technology to explore for various biomedical applications including diagnostics, drug discovery, drug delivery, and sequencing.
Fabrication
However, in the development of any microfluidic device, the fabrication material used is key. The topographical features of devices (e.g. channels, chambers, pillars) have microscale dimensions, introducing a fabrication challenge. Polydimethylsiloxane (PDMS) is the most widely used polymer in the fabrication of microfluidics for biological applications, especially in academic research. It is a biocompatible and transparent material, fabricated through the method of soft lithography. Thanks to PDMS, microfluidics has become more widespread within research and demonstrated the benefits of miniaturization for testing and analysis. Most notably for biomedical applications, PDMS has provided innovators with the ability to construct 3D in vitro environments. However, it may display limitations regarding the absorption of small molecules and poor hydrophilicity, even after surface functionalization [22]. The multi-step fabrication and assembly process of PDMS devices limits the prototyping and scale-up of devices, which is important for scientific publications and product development. Furthermore, the reproducibility of microscale features is poor and the production of thin films from PDMS is technically difficult.
Consequently, it is extremely important to select the correct material for device fabrication. Each material currently available in microfluidics, provides different advantages. In the case of industry, innovators lean towards the use of thermoplastics, such as COC, polycarbonate, or polystyrene. This class of materials is easy to scale up and cost-effective, and harbour the important biocompatibility and transparency properties that biotechnology applications require. Nonetheless, small-scale device fabrication or prototyping remains expensive, keeping it out of reach from most academics. Thermoplastics also often employ harsh chemical and temperature bonding treatments, restricting the surface functionalization of devices.
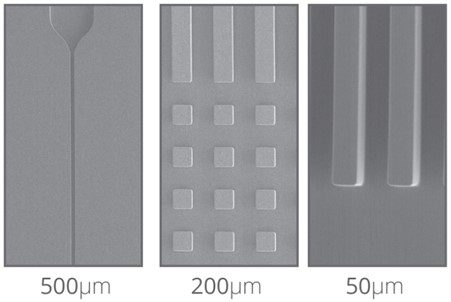
Figure 7. Through fabrication by hot embossing, Eden Tech’s Flexdym™ can achieve sub micrometer resolution and high reproducibility of features from 10 µm.
The current lack of materials capable of extending device fabrication from the initial stages all the way to mass production means imposes limitations on the field of microfluidics. New solutions must be proposed to overcome this. For example, Eden Tech’s Flexdym™ polymer is a thermoplastic elastomer, which combines the advantages of both PDMS and thermoplastics, while providing scale-up opportunities.
The Flexdym is molded through the hot embossing technique, where temperature and pressure are used to transfer micron features from the mold to the material. With this technique, one can achieve sub-micrometer resolution features with high reproducibility. Figure 7 shows some microscale topographical features molded in Flexdym™. Fabrication by hot embossing allows for rapid device prototyping (i.e. scale of minutes) at a higher resolution than PDMS soft-lithography.
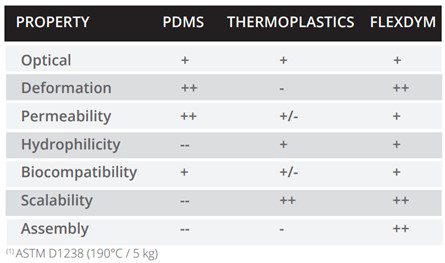
One of the key applications of Flexdym is in 3D cell culture and organ-on-chip devices. As we have covered, in these applications it is important to apply mechanical strain to the culture through stretching, and the substrate must be flexible. With an elongation of 720% and availability in 250 µm thin sheets, Flexdym™ can provide an elastic surface for this application. Both the fabrication and material properties offer advantages over traditional microfluidic materials for cell culture applications, highlighted in Table 1.
Table 1. Comparison of material properties for traditional microfluidic polymers PDMS and thermoplastics compared to Eden Tech Flexdym™ material
References
[1] Dunn GA, Heath JP. A new hypothesis of contact guidance in tissue cells. Exp Cell Res. 1976 Aug;101(1):1-14. doi: 10.1016/0014-4827(76)90405-5. PMID: 182511.
[2] Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010 Sep;31(27):6941-6951. doi: 10.1016/j.biomaterials.2010.05.056. Epub 2010 Jun 19. PMID: 20638973; PMCID: PMC2908986.
[3] Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015 Mar 11;16(3):5517-27. doi: 10.3390/ijms16035517. PMID: 25768338; PMCID: PMC4394490.
[4] Li Y, Huang G, Zhang X, Wang L, Du Y, Lu TJ, Xu F. Engineering cell alignment in vitro. Biotechnol Adv. 2014 Mar-Apr;32(2):347-65. doi: 10.1016/j.biotechadv.2013.11.007. Epub 2013 Nov 22. PMID: 24269848.
[5] Zhao Y, Zeng H, Nam J, Agarwal S. Fabrication of skeletal muscle constructs by topographic activation of cell alignment. Biotechnol Bioeng. 2009 Feb 1;102(2):624-31. doi: 10.1002/bit.22080. PMID: 18958861; PMCID: PMC3825623.
[6] Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40(4):317-30. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. PMID: 9712262.
[8] Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012 Jun 21;12(12):2165-74. doi: 10.1039/c2lc40074j. Epub 2012 Mar 20. PMID: 22434367.
[9] Zamprogno P, Wüthrich S, Achenbach S, Thoma G, Stucki JD, Hobi N, Schneider-Daum N, Lehr CM, Huwer H, Geiser T, Schmid RA, Guenat OT. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun Biol. 2021 Feb 5;4(1):168. doi: 10.1038/s42003-021-01695-0. PMID: 33547387; PMCID: PMC7864995.
[10] Lee AA, Graham DA, Dela Cruz S, Ratcliffe A, Karlon WJ. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J Biomech Eng. 2002 Feb;124(1):37-43. doi: 10.1115/1.1427697. PMID: 11871603.
[11] Kohn JC, Zhou DW, Bordeleau F, Zhou AL, Mason BN, Mitchell MJ, King MR, Reinhart-King CA. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J. 2015 Feb 3;108(3):471-8. doi: 10.1016/j.bpj.2014.12.023. PMID: 25650915; PMCID: PMC4317546.
[12] Lee J, Estlack Z, Somaweera H, Wang X, Lacerda CMR, Kim J. A microfluidic cardiac flow profile generator for studying the effect of shear stress on valvular endothelial cells. Lab Chip. 2018 Sep 26;18(19):2946-2954. doi: 10.1039/c8lc00545a. PMID: 30123895.
[13] Tovar-Lopez F, Thurgood P, Gilliam C, et al. A Microfluidic System for Studying the Effects of Disturbed Flow on Endothelial Cells. Front Bioeng Biotechnol. 2019;7:81. Published 2019 Apr 17. doi:10.3389/fbioe.2019.00081
[14] U. M. Sonmez, Y. Cheng, S. C. Watkins, B. L. Roman and L. A. Davidson, Lab Chip, 2020, 20, 4373 DOI: 10.1039/D0LC00738B
[15] Charest JL, García AJ, King WP. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials. 2007 Apr;28(13):2202-10. doi: 10.1016/j.biomaterials.2007.01.020. Epub 2007 Jan 13. PMID: 17267031.
[16] Anene-Nzelu CG, Peh KY, Fraiszudeen A, Kuan YH, Ng SH, Toh YC, Leo HL, Yu H. Scalable alignment of three-dimensional cellular constructs in a microfluidic chip. Lab Chip. 2013 Oct 21;13(20):4124-33. doi: 10.1039/c3lc50730k. Epub 2013 Aug 23. PMID: 23969512.
[17] Dalby MJ, Gadegaard N, Riehle MO, Wilkinson CD, Curtis AS. Investigating filopodia sensing using arrays of defined nano-pits down to 35 nm diameter in size. Int J Biochem Cell Biol. 2004 Oct;36(10):2005-15. doi: 10.1016/j.biocel.2004.03.001. PMID: 15203114.
[18] Nematollahi M, Hamilton DW, Jaeger NJ, Brunette DM. Hexagonal micron scale pillars influence epithelial cell adhesion, morphology, proliferation, migration, and cytoskeletal arrangement. J Biomed Mater Res A. 2009 Oct;91(1):149-57. doi: 10.1002/jbm.a.32202. PMID: 18773428.
[19] Agrawal G, Aung A, Varghese S. Skeletal muscle-on-a-chip: an in vitro model to evaluate tissue formation and injury. Lab Chip. 2017 Oct 11;17(20):3447-3461. doi: 10.1039/c7lc00512a. PMID: 28871305; PMCID: PMC6296378.
[20] Ahmed WW, Wolfram T, Goldyn AM, Bruellhoff K, Rioja BA, Möller M, Spatz JP, Saif TA, Groll J, Kemkemer R. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials. 2010 Jan;31(2):250-8. doi: 10.1016/j.biomaterials.2009.09.047. Epub 2009 Sep 26. PMID: 19783042.
[21] Lam RH, Sun Y, Chen W, Fu J. Elastomeric microposts integrated into microfluidics for flow-mediated endothelial mechanotransduction analysis. Lab Chip. 2012 Apr 24;12(10):1865-73. doi: 10.1039/c2lc21146g. Epub 2012 Mar 21. PMID: 22437210; PMCID: PMC4120067.
[22] McMillan, A.H.; Thomée, E.K.; Dellaquila, A.; Nassman, H.; Segura, T.; Lesher-Pérez, S.C. Rapid Fabrication of Membrane-Integrated Thermoplastic Elastomer Microfluidic Devices. Micromachines 2020, 11, 731.
The Author

Gwen Newman, Msc.
PhD Candidate, Eden Medtech R&D