HIGH-VOLUME, COMPACT &
BIOMIMETIC MICROFLUIDIC ELECTROLYZER
GREEN HYDROGEN
FOR HIGH-EFFICIENCY RENWEABLE ENERGY PRODUCTION
A path to decarbonised energy
GREEN HYDROGEN
Hydrogen is a central pillar of the energy transformation required to limit global warming to two degrees Celsius. To achieve the two-degree scenario, the world will need to make dramatic changes year after year and decrease energy-related CO2 emissions.
Hydrogen can play a major role in this transformation. By adapting hydrogen production to renewable energy sources, it can enable large-scale integration of renewable energy and power generation. Today, there is an urgent need to develop an alternative source as a substitute for the non-renewable resources.
Current Situation
Decrease of CO2 emissions by 60% until 2050

Population is expected to be 9.8 billion in 2050

About 81% of energy is produced from conventional energy resource.
Demand for hydrogen has grown by around 70M tons per year (MtH2/yr).
State of art on water electrolysis
Hydrogen offers great opportunities for energy storage, however its industrialization should shifted to a new paradigm. Currently, hydrogen is almost entirely supplied from fossil fuels, with 6% of global natural gas and 2% of global coal going to hydrogen production. As a consequence, production of hydrogen is responsible for carbon dioxide (CO2) emissions of around 830 million tonnes of carbon dioxide per year. That’s why new methods are being studied, one of them is hydrogen production through a process called electrolysis of water.
In this renewable electrical process, energy is used by an electrolyzer to split water into hydrogen and oxygen. The potential of the technology is groundbreaking, but to date, the alkaline electrolyzers face a number of limitations. The highly sophisticated and fragile membranes are often made of materials difficult to source, making the systems expensive in capital and operational costs.
Commonly, alkaline electrolyzers have electrode areas as high as 3 square metres (m²). They operate with high concentrate KOH electrolyte, robust ZrO2 based diaphragms and nickel (Ni) coated stainless-steel for the electrodes. The ionic charge carrier is the hydroxyl ion OH-, with KOH and water permeating through the porous structure of the diaphragm to provide functionality for the electrochemical reaction. This allows for avoiding the intermixing of the produced gases (Hydrogen and Oxygen) that are dissolved in the electrolyte, limiting lower power-operating range and the ability to operate at higher pressure levels.
Our Solution
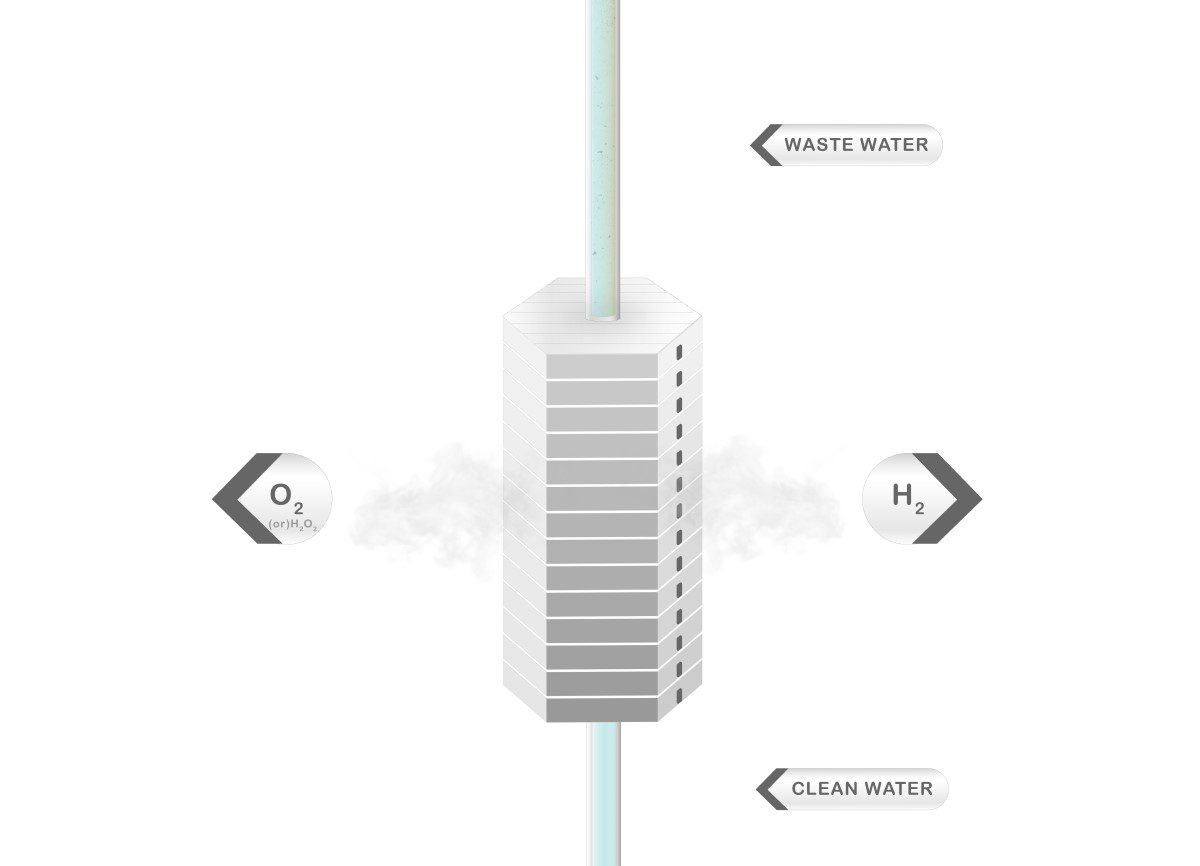
Eden Tech’s solution is based on two approaches: removing the diaphragm and permitting higher current densities using the support of microfluidic and biomimetism concepts.
In the reaction, two molecules of water are decomposed and hydrogen evolves on the cathode. On the anode, oxygen evolves and at the same time one molecule of water is regenerated. As a result, one molecule of water is decomposed and another molecule of water moves to the cathode.
Overvoltage loss is due to “resistance” by the chemical reaction rate. To drive the chemical reaction of electrolysis, extra energy is required in addition to the reversible potential which corresponds to a zero reaction rate. Ohmic loss is mainly caused by electric resistance of electrolyte, which can be reduced by shortening the distance between anode and cathode. Ohmic loss is also caused by electric resistance of circuitry. Both overvoltage and ohmic loss increase with the increasing current density, hence increase of cell voltage and, therefore, increase of electric power to make hydrogen. However, at high current density, the activation overvoltage becomes negligible compared to ohmic losses.
microfluidics approach for an optimal electrolysis
In the context of a microfluidic system, resistances associated with the anode and cathode are not part of the optimization process.
Microfluidics and its micron-sized channels reduces greatly the resistance that leads to the ohmic losses in the electrolizer and most importantly, it allows operation at higher current densities without generating counterproductive voltage losses. With the reduction of the channel size, it becomes possible to explore the use of less-than-ideal electrolyte while still reducing the electrolyte resistance.
Why is it important ?
Based on the reaction stoichiometry, for every kg of hydrogen produced, 9 kg of water must be consumed. Therefore, 2.3 Gt of hydrogen requires 20.5 Gt, or 20.5 billion m3, per year of freshwater, which accounts for only 1.5 ppm of Earth’s available freshwater. Accessible freshwater makes up just less than 1% of the planet’s water, and it is best to avoid creating any additional burden on freshwater usage, especially in areas where drinking water is difficult to attain. With microfluidic hydrogen production, seawater and wastewater become suitable candidates as sustainable electrolytes, protecting our freshwater supplies from unnecessary use. Moreover, green hydrogen energy doesn’t produce any greenhouse gas emissions and is considered a clean energy source. It contributes to reducing air pollution, decreasing dependence on fossil fuels, and mitigating the effects of climate change while cleaning wastewater. Green Hydrogen has a great potential to be a key component in a sustainable energy system.
Discover MacGhyver Consortium
MacGHyver is the acronym for the Microfluidic wAstewater treatment and Creation of Green HYdrogen Via Electrochemical Reactions. MacGhyver produces green hydrogen from wastewater using innovation in high-volume microfluidics, non-CRM electrodes, and electrochemical compression. This project has received funding from the European Innovation Council (EIC) under grant agreement No 101069981.
About Us
Eden Tech is a company reinventing microfluidics with user-friendly and scalable solutions. Our products are aimed at pushing forward both academic and industrial innovation.
Contact Us
HIGH-VOLUME, COMPACT &
BIOMIMETIC MICROFLUIDIC ELECTROLYZER
GREEN HYDROGEN
FOR HIGH-EFFICIENCY RENEWABLE ENERGY PRODUCTION
GREEN HYDROGEN
A path to decarbonised energy
Hydrogen is a central pillar of the energy transformation required to limit global warming to two degrees Celsius. To achieve the two-degree scenario, the world will need to make dramatic changes year after year and decrease energy-related CO2 emissions.
Hydrogen can play a major role in this transformation. By adapting hydrogen production to renewable energy sources, it can enable large-scale integration for power generation. Today, there is an urgent need to develop an alternative source as a substitute for the non-renewable resources.
Current Situation

Target to reduce CO2 emissions from industries by 81% by 2050

Fossil fuels currently supply 80% of the world's energy

Population is expected to be 9.8 billion in 2050

Demand for hydrogen has grown by around 70M tons per year (MtH2/yr).
State of the art on Hydrogen production
Hydrogen offers great opportunities for energy storage, however its industrialization should shift to a new paradigm. Currently, hydrogen is almost entirely supplied from fossil fuels, with 6% of global natural gas and 2% of global coal going to hydrogen production. That’s why new methods are being studied, one of them is hydrogen production through a process called electrolysis of water.
In this process, energy is used by an electrolyzer to split water into hydrogen and oxygen. The potential of the technology is groundbreaking, but to date, the alkaline electrolyzers face a number of limitations. The highly sophisticated and fragile membranes are often made of materials difficult to source, making the systems expensive in capital and operational costs.
Commonly, alkaline electrolyzers have electrode areas as high as 3m². They operate with high concentrate KOH electrolyte, robust ZrO2 based diaphragms and nickel (Ni) coated stainless-steel for the electrodes. The ionic charge carrier is the hydroxyl ion OH-, with KOH and water permeating through the porous structure of the diaphragm to provide functionality for the electrochemical reaction. This allows the intermixing of the produced gases (Hydrogen and Oxygen) that are dissolved in the electrolyte, limiting lower power-operating range and the ability to operate at higher pressure levels.
Our Solution
Eden Tech’s solution is based on two approaches: removing the diaphragm and permitting higher current densities using the support of microfluidic and biomimetism concepts.
In the reaction, two molecules of water are decomposed and hydrogen evolves on the cathode. On the anode, oxygen evolves and at the same time one molecule of water is regenerated. As a result, one molecule of water is decomposed and another molecule of water moves to the cathode.
Overvoltage loss is due to “resistance” by the chemical reaction rate. To drive the chemical reaction of electrolysis, extra energy is required in addition to the reversible potential which corresponds to a zero reaction rate. Ohmic loss is mainly caused by electric resistance of electrolyte, which can be reduced by shortening the distance between anode and cathode. Ohmic loss is also caused by electric resistance of circuitry. Both overvoltage and ohmic loss increase with the increasing current density, hence increase of cell voltage and, therefore, increase of electric power to make hydrogen. However, at high current density, the activation overvoltage becomes negligible compared to ohmic losses.

microfluidics approach for an optimal electrolysis
In the context of a microfluidic system, resistances associated with the anode and cathode are not part of the optimization process.
Microfluidics and its micron-sized channels reduces greatly the resistance that leads to the ohmic losses in the electrolizer and most importantly, it allows operation at higher current densities without generating counterproductive voltage losses. With the reduction of the channel size, it becomes possible to explore the use of less-than-ideal electrolyte while still reducing the electrolyte resistance.
Why is it important?
Based on the reaction stoichiometry, for every kg of hydrogen produced, 9 kg of water must be consumed. Therefore, 2.3 Gt of hydrogen requires 20.5 Gt, or 20.5 billion m3, per year of freshwater, which accounts for only 1.5 ppm of Earth’s available freshwater. Accessible freshwater makes up just less than 1% of the planet’s water, and it is best to avoid creating any additional burden on freshwater usage, especially in areas where drinking water is difficult to attain. With microfluidic hydrogen production, seawater and wastewater become suitable candidates as sustainable electrolytes, protecting our freshwater supplies from unnecessary use. Moreover, green hydrogen energy doesn’t produce any greenhouse gas emissions and is considered a clean energy source. It contributes to reducing air pollution, decreasing dependence on fossil fuels, and mitigating the effects of climate change while cleaning wastewater. Green Hydrogen has a great potential to be a key component in a sustainable energy system.
Discover MacGhyver Consortium
MacGHyver is the acronym for the Microfluidic wAstewater treatment and Creation of Green HYdrogen Via Electrochemical Reactions. MacGhyver produces green hydrogen from wastewater using innovation in high-volume microfluidics, non-CRM electrodes, and electrochemical compression. This project has received funding from the European Innovation Council (EIC) under grant agreement No 101069981.
About Us
Eden Tech is a company reinventing microfluidics with user-friendly and scalable solutions. Our products are aimed at pushing forward both academic and industrial innovation.




