Acoustofluidic Microfluidics: A PDMS-Free Innovation
Introduction: Moving Beyond PDMS in Acoustofluidics Here
Acoustofluidics is a hybrid field combining acoustics and microfluidics, and has become a cornerstone in biomedical research, enabling precise fluid manipulation using sound waves. Among its most impactful technologies are Lateral Cavity Acoustic Transducers (LCATs), which leverage microstreaming to perform tasks like cell sorting, gene delivery, and molecular mixing. However, conventional LCATs are typically fabricated using polydimethylsiloxane (PDMS) a material plagued by high gas permeability, solvent incompatibility, and limited scalability.
To address these issues, a recent thesis from UC Irvine presents a PDMS-free alternative using Flexdym, a thermoplastic elastomer optimized for microfluidics. The study, led by Edilber Palacios under the supervision of Prof. Abraham Lee, systematically demonstrates how Flexdym outperforms PDMS in acoustofluidic applications while enabling scalable, reusable, and stable microfluidic platforms.
What Are LCATs and Why Do They Matter?
Lateral Cavity Acoustic Transducers (LCATs) are microfluidic structures that incorporate air-filled side cavities along microchannels. When excited by acoustic waves, these cavities generate vortices through controlled oscillations of the air–liquid interface, allowing for non-contact fluid handling.
LCATs have proven essential in:
High-throughput cell separation [16]
DNA shearing for genetic analysis [17]
Intracellular delivery in gene therapy [20]
Rapid immunodiagnostics [18]
Whole blood sorting and in situ labeling [19]
Despite their promise, PDMS-based LCATs face critical drawbacks due to the material’s air permeability and chemical limitations. This affects long-term performance and restricts their adoption in clinical or commercial settings.
Flexdym: A PDMS-Free Material for Scalable Microfluidics
Flexdym, a thermoplastic elastomer alternative to PDMS, addresses many of these limitations:
Low gas permeability, ensuring stable air–liquid interfaces
Chemical resistance to solvents like ethanol and methanol
Minimal absorption of small molecules and dyes [28]
Scalable fabrication via hot embossing and injection molding
Multiple bonding options, including reversible conformal bonding
Unlike PDMS, Flexdym supports hot embossing, allowing for rapid, reproducible, and scalable fabrication of microfluidic chips—ideal for academic labs and industrial use alike.
Key Findings: Flexdym-Based LCATs in Acoustofluidics
The thesis rigorously compares PDMS and Flexdym in identical LCAT setups, revealing significant performance enhancements with Flexdym:
1. Superior Air–Liquid Interface Stability
Flexdym devices maintained air–liquid interfaces for over 2 hours under acoustic actuation, while PDMS devices degraded after ~40–60 minutes. This is attributed to Flexdym’s lower oxygen permeability, which retains air in lateral cavities and ensures consistent vortex generation.
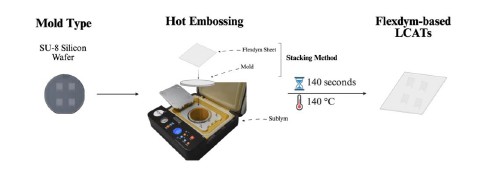
2. Scalable Fabrication via Hot Embossing
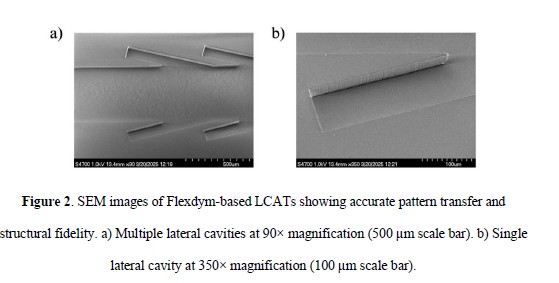
Using a Sublym Hot Embosser, Flexdym LCATs were fabricated in less than 3 minutes with high fidelity, as confirmed by SEM imaging. Unlike soft lithography with PDMS, this method supports both rapid prototyping and mass production.
3. Bonding Versatility and Reusability
Conformal bonding allowed for leak-free operation with reversible chip assembly—ideal for modular devices and iterative experiments. In contrast, PDMS requires irreversible plasma bonding, limiting flexibility.
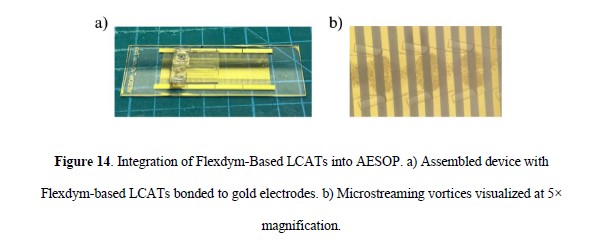
4. Integration into the AESOP Platform
Flexdym-based LCATs were successfully integrated into the Acoustic-Electric Shear Orbiting Poration (AESOP) platform, enabling electric field–assisted gene delivery. CD47 knockout in T cells showed a 22% efficiency, comparable to PDMS-based devices (18%) but with improved stability and reusability.

Implications for the Acoustofluidic Community
This study marks the first systematic evaluation of Flexdym in acoustofluidics, offering a new PDMS-free path forward for long-term, high-performance microfluidic systems. Flexdym’s unique combination of fabrication speed, surface stability, and biocompatibility makes it especially attractive for:
Lab-on-a-chip devices
Portable diagnostics
Cell engineering platforms
High-throughput screening systems
For researchers exploring acoustic microstreaming or seeking long-term vortex stability, Flexdym offers a practical, scalable, and sustainable solution.
Conclusion: Rethinking Acoustofluidics with Flexdym
As acoustofluidics evolves toward real-world biomedical applications, material limitations must be addressed. This UC Irvine study demonstrates that Flexdym is not just a viable alternative to PDMS; it is a superior platform for building the next generation of acoustofluidic devices.
With better interface stability, scalable manufacturing, and multi-modal compatibility, Flexdym is setting a new standard for acoustofluidic microfluidics.
References
Key references from the thesis include:
[6] Tovar, A. R., & Lee, A. P. (2009). Lateral cavity acoustic transducer. Lab on a Chip, 9, 41–43. https://doi.org/10.1039/B812435C
[16] Nivedita, N., Luan, D., Tung, S., & Papautsky, I. (2017). A high-throughput microfluidic platform for size-selective enrichment of cell populations in tissue and blood samples. Analyst, 142(15), 2558–2571. https://doi.org/10.1039/C7AN00290D
[17] Okabe, Y., & Lee, A. P. (2014). LCAT DNA shearing. Journal of Laboratory Automation, 19(2), 163–170. https://doi.org/10.1177/2211068213495546
[18] Garg, N., Li, Y., Chen, H., Zhang, Y., & Wang, S. (2019). Rapid immunodiagnostics of multiple viral infections in an acoustic microstreaming device with serum and saliva samples. Lab on a Chip, 19, 1524–1533. https://doi.org/10.1039/C8LC01303A
[19] Garg, N., Li, Y., Chen, H., & Wang, S. (2018). Whole-blood sorting, enrichment, and in situ immunolabeling of cellular subsets using acoustic microstreaming. Microsystems & Nanoengineering, 4, Article 17085. https://doi.org/10.1038/micronano.2017.85
[20] Aghaamoo, M., Yen, G. D., Akhavan Tabatabaei, R., Lee, J., & Lee, A. P. (2021). High-throughput and dosage-controlled intracellular delivery of large cargos by an acoustic-electric micro-vortices platform. Advanced Science, 8(21), 2102021. https://doi.org/10.1002/advs.202102021
[28] Lachaux, J., Boutry, C. M., Thibert, C., Taffin, C., Schneider, B., Camon, H., & Gabriele, S. (2017). Thermoplastic elastomer with advanced hydrophilization and bonding performances for rapid (30 s) and easy molding of microfluidic devices. Lab on a Chip, 17, 2581–2594. https://doi.org/10.1039/C7LC00488E
[31] Eden Microfluidics. (n.d.). Flexdym for fast & easy microfluidic device fabrication. https://eden-microfluidics.com/news-events/flexdym-for-fast-easy-microfluidic-device-fabrication
📄 Full thesis available at: Flexdym-based LCATs by Edilber Palacios (UC Irvine)

