Droplet-Based Microfluidics: A Design Guideline Introduction
Introduction
Droplet-based microfluidics has emerged as a powerful tool for applications in biotechnology, chemistry, and material sciences. By leveraging the ability to generate and manipulate discrete droplets in immiscible phases, this technique offers high throughput, precise control over reaction conditions, and minimal sample consumption. It’s important to highlight that the design of droplet-based microfluidic devices, however, requires careful consideration of various parameters, including channel geometry, flow conditions, and material selection. This guide outlines key design principles and provides insights into optimizing droplet generation and manipulation.
Fundamental Principles of Droplet-Based Microfluidics
Droplet microfluidics has revolutionized research by enabling high-throughput experimentation and precise biochemical analysis. By generating and manipulating tiny, monodisperse droplets as isolated microreactors, this technology has transformed fields such as enzyme kinetics, synthetic biology, and single-cell analysis. It allows rapid screening of biomolecules, facilitating drug discovery and personalized medicine while minimizing reagent consumption. Widely used in single-cell RNA sequencing and immunoassays, droplet microfluidics provides unparalleled insight into cellular heterogeneity. Despite technical challenges, advancements in chip fabrication and digital droplet technologies continue to expand its applications, solidifying its role as a key tool in biotechnology and biomedical research.
Droplet-Based Microfluidics Generation Mechanisms
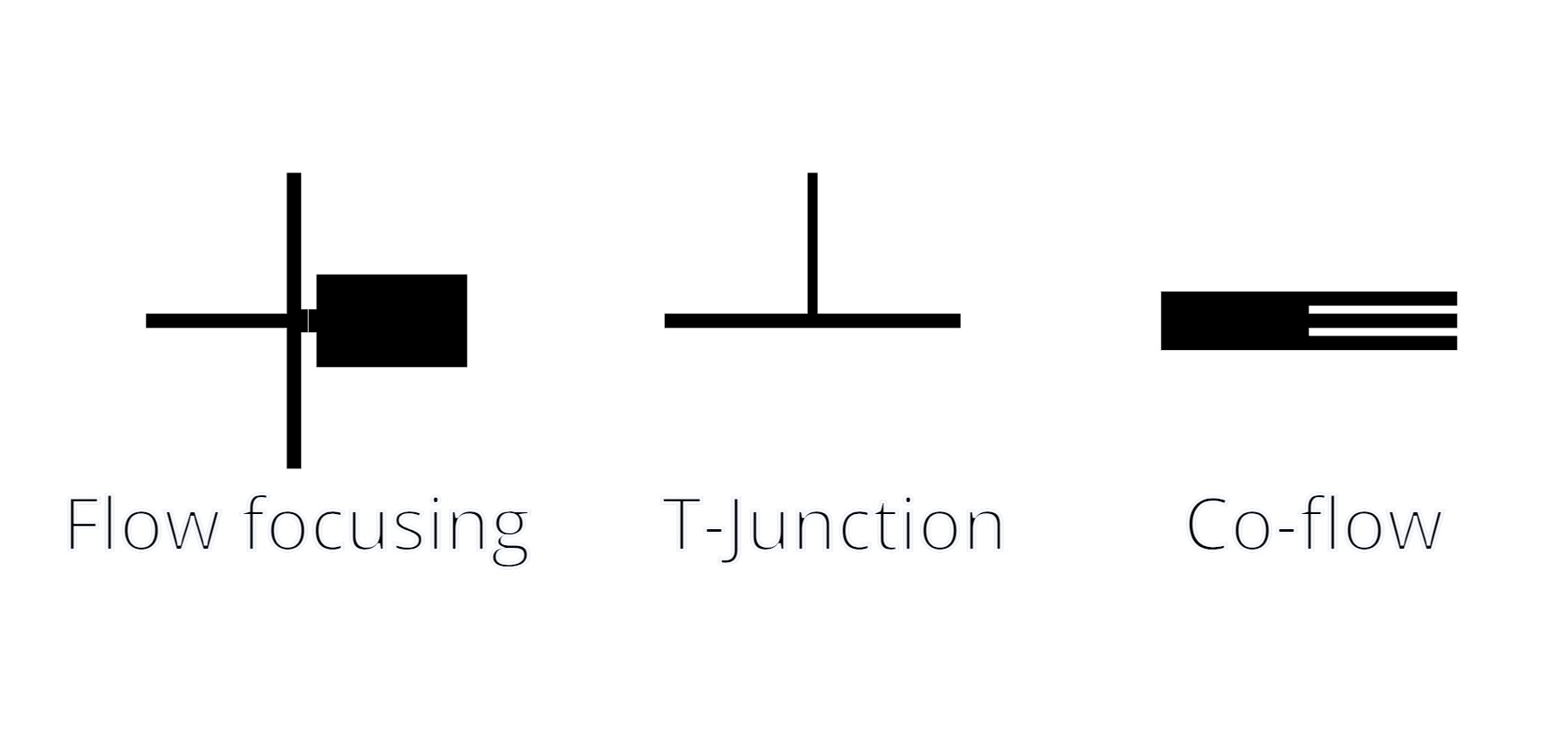
There are 3 main geometries for droplet formation, each suited to specific applications:
-
- Flow Focusing: A dispersed phase is injected into a cross-junction where it is pinched by a continuous phase, forming droplets (Anna et al., 2003).
-
- T-Junction: A simple design where two immiscible phases intersect perpendicularly, producing droplets through shear forces (Thorsen et al., 2001).
-
- Co-Flow: Droplets form within a continuous phase due to controlled shear stress along a coaxial geometry (Umbanhowar et al., 2000).
Key Design Considerations for Droplet-Based Microfluidics
When designing an effective droplet-based microfluidic device, several critical parameters must be considered, including geometry, material selection and its chemical properties, flow rate, capillary number, and the surfactant used. However, for the scope of this article, we will not cover all of these aspects in detail.
1. Channel Geometry
Channel dimensions significantly influence droplet size and stability. Smaller channels (typically 10-100 μm in width) result in more stable and monodisperse droplets. The aspect ratio (height-to-width) also plays a role in controlling flow behavior and droplet breakup.
2. Wettability and Surface Coating
Material selection affects surface properties and interactions between phases. Hydrophobic materials (e.g., Flexdym with surface treatment, see the Application note related) are preferred for water-in-oil systems, while hydrophilic coatings (e.g., PEGylated surfaces) facilitate oil-in-water droplets (Abate et al., 2008).
3. Flow Rate Control and Capillary Number
The ratio of viscous to interfacial forces, represented by the Capillary number (Ca = ηU/σ), governs droplet formation. Flow rate ratios between the continuous and dispersed phases must be optimized to achieve stable dripping or jetting regimes (Christopher & Anna, 2007).
4. Emulsification and Stability
In droplet-based microfluidics, generating stable water-in-oil droplets is critical for applications like digital PCR and single-cell analysis. Emulseo’s surfactants, such as FluoSurf™-C, FluoSurf™-O, and FluoSurf™-S, play a pivotal role by reducing interfacial tension and preventing droplet coalescence, even under thermocycling conditions. These biocompatible surfactants enable the creation of monodisperse droplets (1–300 μm) in fluorinated oils like Fluo-Oil™ 135 (Novec™ 7500 alternative) and Fluo-Oil™ 200 (Fluorinert™ FC-40 alternative), ensuring stability and good leakage control for high-throughput analyses.
Complementing these, Emulseo’s hydrophobic surface treatments (Fluo-ST1™ and Fluo-ST3™) enhance droplet performance by covalently bonding to microfluidic channel walls.
Advanced Droplet-Based Microfluidics Manipulation Techniques
Droplets within microfluidic systems can be manipulated through various design strategies to achieve specific functionalities. These techniques allow for precise control of droplet behavior, enabling a wide range of applications in biological and chemical research.
1. Transport and Sorting
The movement of droplets within microchannels is dictated by the surrounding carrier fluid. Lubrication films forming between droplets and channel walls influence flow resistance and velocity (Baroud et al., 2010). Sorting techniques such as dielectrophoresis (DEP), fluorescence-activated sorting, and inertial focusing allow for the selective isolation of droplets based on size, composition, or fluorescence properties (Link et al., 2006).
2. Encapsulation and Controlled Reactions
Encapsulation of biological or chemical reagents enables single-cell analysis, high-throughput screening, and controlled reactions. Methods such as microvalves and pico-injection allow for precise reagent addition into droplets, ensuring reproducibility and accuracy (Theberge et al., 2010).
3. Merging, Splitting, and Dilution
Droplet merging and splitting techniques are essential for multi-step reactions and combinatorial assays. Baroud et al. (2010) describe passive and active merging strategies, including:
-
-
Decompression merging: Expansion-contraction channels bring droplets into close contact, promoting natural coalescence.
-
-
-
Electro-fusion: An electric field destabilizes the liquid film between droplets, inducing rapid merging.
-
-
-
Thermal activation: Localized heating reduces interfacial tension, enabling droplet fusion.
-
Geometric modifications to microfluidic channels enable controlled droplet splitting for dilution or parallel processing (Bremond et al., 2008).
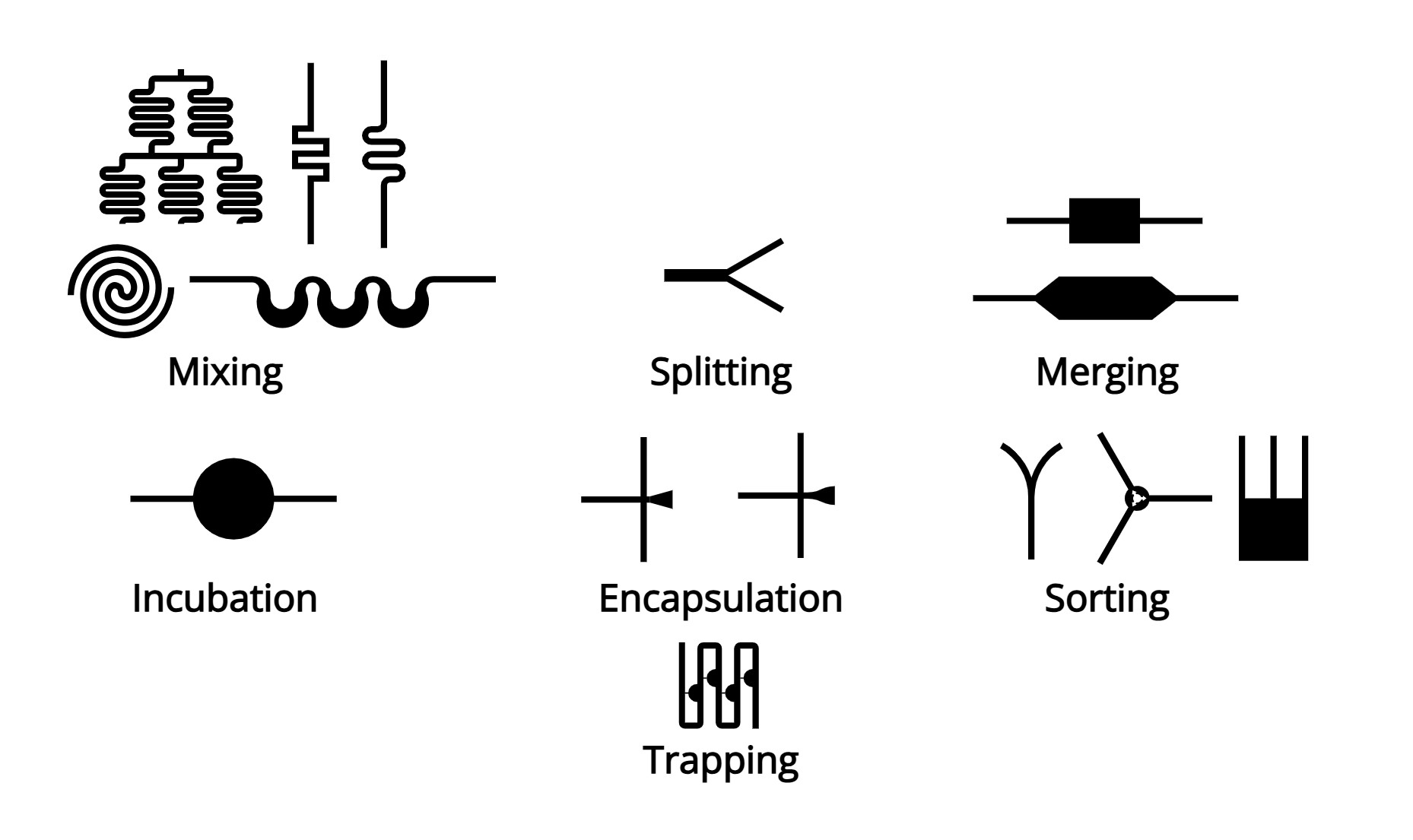
4. Additional Droplet Manipulations
Different droplet manipulation techniques can be integrated within microfluidic chips to facilitate complex workflows (Moragues et al., 2023):
-
-
Mixing: Enhancing reagent interaction within droplets for efficient chemical reactions.
-
-
-
Incubation: Maintaining droplets under specific environmental conditions to support biological or chemical processes.
-
-
-
Injection: Adding additional reagents into droplets using precision pico-injection.
-
-
-
Trapping: Immobilizing droplets in designated areas for extended observation or analysis.
-
-
-
Droplet Sensing: Utilizing optical, electrical, or magnetic detection methods for real-time analysis of droplet contents.
-
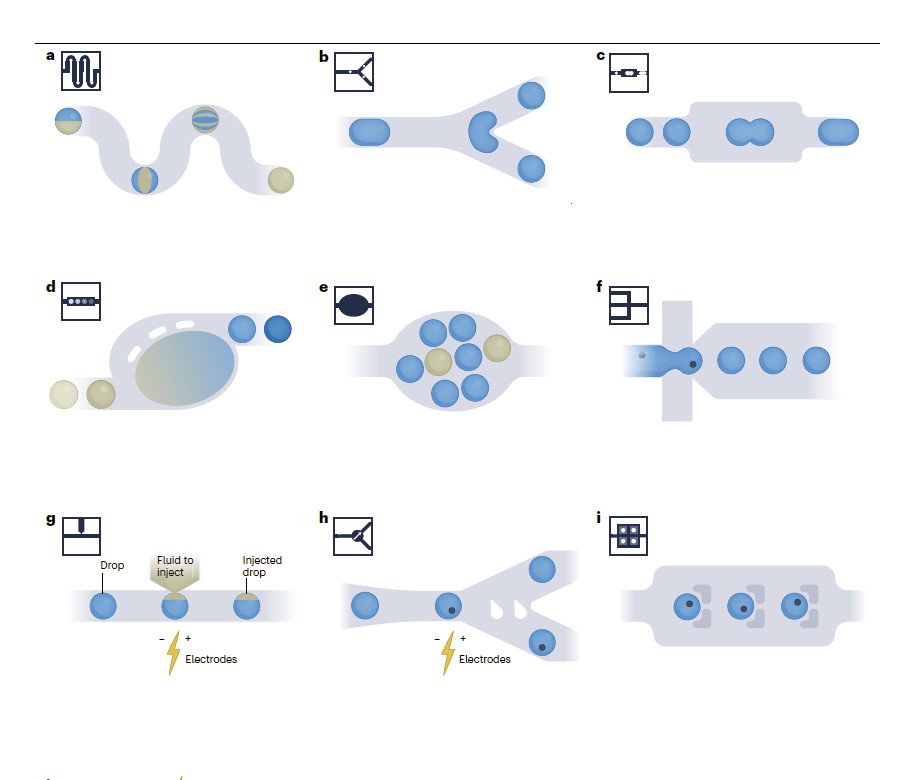
Microfluidics module for droplet Manipulation, Moragues et al. (2023), Nature reviews
FLUI'DEVICE: A Revolutionary Tool for Droplet-Based Microfluidic Design
Designing droplet-based microfluidic devices is complex, and requires precise expertise in geometry and iteration. FLUI’DEVICE simplifies this process by offering an intuitive, web-based platform that allows users to design, simulate, and export microfluidic devices effortlessly.
Key Benefits of Using FLUI’DEVICE for Droplet-Based Microfluidics:
-
- User-Friendly Interface: No CAD experience required—drag-and-drop modules allow rapid prototyping. Fast and easy resizing.
-
- Pre-Designed Droplet Modules: Access ready-to-use flow-focusing, T-junction, co-flow designs, and droplet manipulation module
-
- Seamless Export Options: Generate STL, DXF, and SVG files for fabrication or direct integration with microfabrication partners.
By integrating FLUI’DEVICE into your workflow, you can accelerate the design cycle, reduce trial-and-error in microfluidic development, and bring innovative droplet-based applications to market faster.
Conclusion
Designing droplet-based microfluidic devices requires careful optimization of multiple parameters, including channel geometry, flow dynamics, and material properties. By understanding the fundamental principles and leveraging recent technological advancements, researchers can develop robust and scalable microfluidic platforms for a wide range of applications. Tools like FLUI’DEVICE further simplify this process, making microfluidic design more accessible and efficient than ever before.
References
-
- Baroud, C. N., Gallaire, F., & Dangla, R. (2010). Dynamics of microfluidic droplets. Lab on a Chip, 10(2032-2045).
-
- Moragues, et al (2023), Droplet-Based Microfluidics, Nature reviews
-
- Anna, S. L., Bontoux, N., & Stone, H. A. (2003). Formation of dispersions using “flow focusing” in microchannels. Applied Physics Letters, 82(3), 364-366.
-
- Baret, J. C. (2012). Surfactants in droplet-based microfluidics. Lab on a Chip, 12(3), 422-433.
-
- Bremond, N., Thiam, A. R., & Bibette, J. (2008). Decompressing emulsion droplets favors coalescence. Physical Review Letters, 100(2), 024501.
-
- Christopher, G. F., & Anna, S. L. (2007). Microfluidic methods for generating continuous droplet streams. Journal of Physics D: Applied Physics, 40(19), R319.
-
- DeMello, A. J. (2006). Control and detection of chemical reactions in microfluidic systems. Nature, 442(7101), 394-402.
-
- Link, D. R., Anna, S. L., Weitz, D. A., & Stone, H. A. (2006). Geometrically mediated breakup of drops in microfluidic devices. Physical Review Letters, 96(13), 134001.
-
- Shen, B., Lu, J. J., & Holst, J. (2014). Droplet microfluidics for single-cell biology. Proceedings of the National Academy of Sciences, 111(10), 3665-3670.
-
- Teh, S. Y., Lin, R., Hung, L. H., & Lee, A. P. (2008). Droplet microfluidics. Lab on a Chip, 8(2), 198-220.
-
- Theberge, A. B., et al. (2010). Microdroplets in microfluidics. Angewandte Chemie, 49(34), 5846-5868.
-
- Thorsen, T., et al. (2001). Dynamic pattern formation. Physical Review Letters, 86(18), 4163.
-
- Umbanhowar, P., et al. (2000). Monodisperse Emulsion Generation via Drop Break off in a Coflowing Stream

