Exploring the Potential of Flexdym in Fabricating MultiU-Int Microfluidic Chips for Deciphering the Immunopathogenesis of Inflammatory Bowel Disease (IBD)
Case Study
The intricate immunopathogenesis of Inflammatory Bowel Disease (IBD) necessitates advanced experimental models to decipher its complex mechanisms. Recent advancements in microfluidic technologies, particularly in the development of intestine-on-chip systems, offer promising avenues for studying IBD. A noteworthy innovation in this domain is the MultiU-Int microfluidic chip, a versatile platform designed to unravel the roles of various immune cell populations in the onset and progression of IBD. Central to this innovation is the use of Flexdym, a thermoplastic elastomer, which plays a crucial role in overcoming the limitations of traditional materials like polydimethylsiloxane (PDMS) in microfluidic chip fabrication.
The Challenge of Modeling IBD
IBD, encompassing Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by chronic inflammation of the gastrointestinal tract. Despite extensive research, the etiology of IBD remains largely elusive, complicating the development of effective therapies. Traditional models, including animal models and ex vivo human tissues, have provided valuable insights but are limited by ethical concerns, species differences, and short lifespan. Consequently, there is a pressing need for physiologically relevant in vitro models that can mimic the human intestinal environment and allow for detailed investigation of the immune responses involved in IBD.
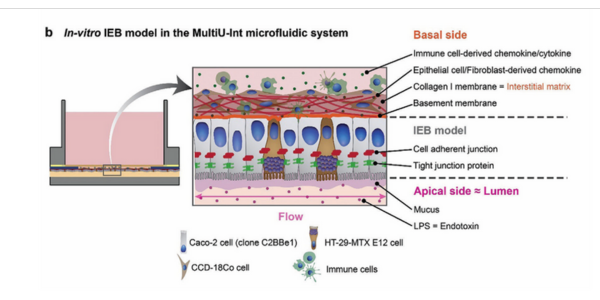
Figure 1. Schematic representation of key structural and cellular components of b) an in vitro IEB model formed within the MultiU-Int microfluidic chip.
Flexdym: A Game-Changer in Microfluidic Chip Fabrication
One of the significant advancements in the field is the development of the MultiU-Int microfluidic chip, which leverages the unique properties of Flexdym. Flexdym is a thermoplastic elastomer known for its excellent mechanical properties, low absorption of small hydrophobic molecules, and ease of fabrication. Unlike PDMS, which is prone to absorption of bioactive molecules and suffers from evaporation issues, Flexdym provides a more stable and reliable material for microfluidic chip fabrication.
In the MultiU-Int system, Flexdym is used to fabricate the bottom layer of the chip, which contains microfluidic channels that replicate the fluidic dynamics of the human intestine. These channels are essential for creating the appropriate shear stress required for the differentiation and maturation of intestinal epithelial cells (IECs). The use of Flexdym allows for rapid prototyping and scalability, making it an ideal material for high-throughput experiments, moreover, its low ad/absorption of small hydrophobic molecules makes Flexdym a more suitable material for the realization of the MultiU-Int system.
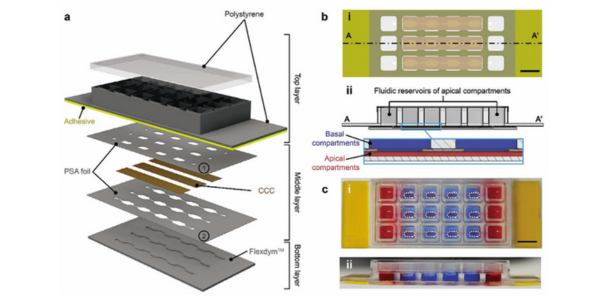
Figure 2. a) Schematic representation of the individual layers of the MultiU-Int microfluidic chip, their arrangement, and materials. b) Schematic cross-sections of the MultiU-Int microfluidic chip showing details of the layer alignment: i) bottom view, and ii) side view. c-i) Top view, and c-ii) side view photographs of the MultiU-Int microfluidic chip. For visualization, apical channels and their reservoirs were filled with red fluid, and the basal compartments were filled with blue fluid. The cell seeding areas on the basal side are marked with white dashed lines. Scale bars: 10 mm.
MultiU-Int: A Novel Intestine-on-Chip System
The MultiU-Int system is designed to model the intestinal epithelial barrier (IEB) with a high degree of physiological relevance. The chip incorporates a collagen I membrane, which acts as a scaffold for IECs, allowing them to grow and differentiate similarly to in vivo conditions.
This membrane is sandwiched between the Flexdym-based bottom layer and a polystyrene top layer, creating a microenvironment that supports the interaction between IECs and immune cells.
What sets the MultiU-Int system apart is its ability to support the independent introduction of different immune cell types and inflammatory stimuli into specific compartments of the chip.
This design enables researchers to study the distinct roles of immune cells, such as macrophages, dendritic cells, and T cells, in the development of intestinal inflammation. By applying inflammatory stimuli, such as lipopolysaccharides (LPS) or pro-inflammatory cytokines, to these compartments, the system can mimic the onset of IBD and allow for the investigation of immune responses in a controlled and systematic manner.
Deciphering Immunopathogenesis with MultiU-Int
The MultiU-Int system has already demonstrated its potential in deciphering the complex interactions between immune cells and the IEB during IBD. For example, in experiments where LPS and interferon-gamma (IFN-γ) were introduced into specific compartments, the system effectively modeled the inflammatory processes observed in IBD.
This setup also facilitated the evaluation of therapeutic interventions, such as the administration of the anti-inflammatory antibody Infliximab, which successfully reduced the levels of tumor necrosis factor-alpha (TNF-α) and other cytokines, mirroring the clinical response in IBD patients.
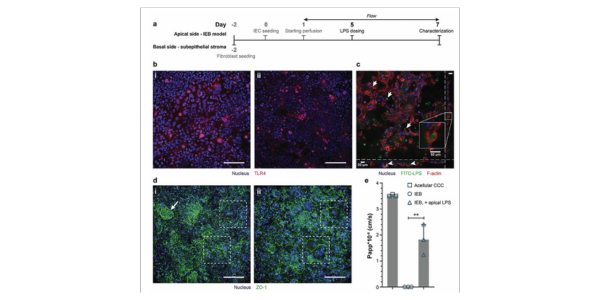
Figure 4. a) Experimental timeline showing the time point and site of LPS administration. b) TLR4 expression in IECs on day 7 i) without and ii) with LPS exposure (10 ng mL−1 LPS), shown by z-stack projection. Scale bars: 100 μm. c) Internalization of FITC-labeled LPS by IECs (arrows) shown by xy (largest insert), xz-, and yz- cross-sections of a z-stack. Cell borders were visualized by F-actin staining. Scale bars: 50 μm. d) IF staining of the IEB model at day 7 under different conditions: i) control IEB model without apical LPS with 3D cellular organization (arrow) and well-established ZO-1, ii) IEB with 10 ng mL−1 LPS on the apical side. The LPS-treated IEB model was underdeveloped in 3D, and giant, multinuclear cells were observed within the barrier while IECs within the control barrier appeared to have small and even cell sizes (dash boxes). Scale bars: 100 μm. e) IEB model permeability for 70 kDa RITC-dextran at day 7, shown as Papp values (n = 3 basal compartments of 3 individual IEB models). Values are means ± SD. Statistical analysis by one-way ANOVA with Tukey–Kramer post hoc test (**: p < 0.01).
Conclusion: The Future of IBD Research
The integration of Flexdym in the fabrication of the MultiU-Int microfluidic chip represents a significant leap forward in the study of IBD. By providing a stable, scalable, and physiologically relevant platform, the MultiU-Int system opens new avenues for understanding the immunopathogenesis of IBD and for testing potential therapeutic strategies. As research continues to evolve, such advanced models will be indispensable in bridging the gap between in vitro studies and clinical applications, ultimately leading to better management and treatment of IBD.
References

